



ELISA studies on bacteria. ELISA results with whole K. pneumoniae, P. mirabilis, and E. coli are shown in Table 1. The patients with AS with active disease had elevated IgA antibodies to K. pneumoniae compared to controls (t = 5.72, p < 0.001) (Figure 1A). IgA anti-Klebsiella antibody levels correlated with both ESR (r = 0.606, p < 0.001) and serum CRP (r = 0.540, p < 0.001). Antibodies of the IgG and IgM class showed no significant elevation to K. pneumoniae. Patients with inactive AS showed no significant elevation in IgG, IgA, or IgM antibody class compared to controls. No antibody elevation of any class was seen against P. mirabilis in active or inactive AS patients compared to controls. Similarly, no elevation in any antibody class against E. coli was seen in active AS or inactive AS compared to controls. Patients with active RA showed elevated IgG (t = 14.10,
Table 1. IgG, IgA, and IgM ELISA antibody (OD units) (mean ± SE) results against whole bacteria (K. pneumoniae, P. mirabilis, and E. coli) in patients and controls. OD^ ± SE.
Active AS (n = 29) |
Inactive AS (n = 23) |
Active RA Probably Active RA (n = 30) (n = 20) |
Controls (n = 50) | ||
Klebsiella pneumoniae |
|||||
IgG |
0.898 ±0.051 |
0.769 ± 0.043 |
0.790 ± 0.040 |
0.556 ± 0.074 |
0.785 ± 0.038 |
IgA |
0.661 ± 0.060* |
O.I 54 ±0.022 |
0.272 ±0.041 |
0.205 ±0.041 |
0.222 ±0.0 19 |
IgM |
0.458 ± 0.037 |
0.357 ± 0.023 |
0.336 ± 0.025 |
0.344 ± 0.029 |
0.334 ±0.0 19 |
Proteus mirabilis |
|||||
IgG |
0.220 ± 0.020 |
0.199*0.024 |
0.752 ± 0.045* |
0.456 ± 0.035* |
0.219 ±0.012 |
IgA |
0.051 ±0.011 |
0.046 ± 0.008 |
0.034 ± 0.006 |
0.035 ± 0.005 |
0.031 ±0.003 |
IgM |
0.105 ±0.011 |
0.102 ±0.012 |
0.144 ±0.014* |
0.1 04 ±0.008 |
0.093 ± 0.005 |
Escherichia coli |
|||||
IgG |
0.730 ± 0.043 |
0.619 ±0.051 |
0.691 ±0.028 |
0.721 ± 0.046 |
0.820 ± 0.025 |
IgA |
0.134 ±0.016 |
0.111 ±0.016 |
0.129 ±0.015 |
0.069 ± 0.008 |
0.1 16 ±0.009 |
IgM |
0.348 ± 0.038 |
0.329 ± 0.034 |
0.408 ± 0.038 |
0.361 ±0.026 |
0.385 ± 0.026 |
* p < 0.001 compared to controls.
110
The Journal of Rheumatology 1997; 24:1
PROTEUS-IgG
Ob)
KLEBSIELLA-IgA
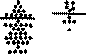
1-50
1-5O
1-25 O D 1-00-
0-75
0-50-
0-25-
0-
1-25-
O D
1-00-
0-75-
0-50'
0-25-
·-Y-
*««*·«
·*···
Controls Active Probably Active
RA ac«ve As
RA
Active RA
Inactive AS
Probably Active
Inactive AS
Controls
Figure 1. IgA Klebsiella antibodies (1 A) and IgG Proteus antibodies (1B) in patients with RA (active and probably active), patients with AS (active and inactive) compared to control subjects. The broken line represents 95% confidence limit of the distribution of.the controls (bars = means).
p < 0.001) and IgM (t = 4.01, p < 0.001) antibodies against
P. mirabilis compared to controls (Figure IB). However,
. IgA antibody was not significantly elevated. Similar results
-. were seen in the probably active RA patients, with IgG anti-
fbody levels elevated compared to controls (t = 8.30, p <
; 0.001), whereas IgA and IgM levels were not. There was no
'significant correlation between IgG anti-Proteus antibody
levels and the acute phase reactants ESR and serum CRP.
Antibodies against K. pneumoniae were not elevated in
active or probably active patients. No elevation in any class
of antibody was seen against E. coll in active or probably
active patients.
ELISA studies on synthetic peptides. ELISA results with 3 synthetic peptides are shown in Table 2. Active AS patients showed significant elevation in all antibody classes against the HLA-B27 peptide compared to controls (IgG; t = 11.94, p < 0.001), (IgA; t = 8.298, p < 0.001), (IgM; t = 7.503, p < 0.001) (Figure 2A). Similar results were seen in IgG (t = 5.908, p < 0.001) and IgA (t = 5.776, p < 0.001) antibodies in the inactive AS patients. There was no significant correlation between antibody levels to HLA-B27 peptide and the acute phase reactants ESR and serum CRP, except between IgG anti-B27 peptide antibody levels and CRP (r = 0.332, p
· Table 2. IgG, IgA, and IgM ELISA antibody (OD units) (mean ± SE) results against peptides (HLA-B27, pullulanase-D, and control) in patients and control subjects. OD,W ± SE.
Active AS (n = 29) |
Inactive AS (n = 23) |
Active RA
|
Probably Active RA (n = 20) |
Controls (n = 50) | |
HLA-B27 peptide |
|||||
IgG |
0.446 ± 0.027* |
0.281 ±0.024* |
0.153 ±0.010 |
0.123+0.012 |
0.159 ±0.010 |
IgA |
0.195 ±0.012* |
0.161 ±0.015* |
0.073+0.010 |
0.036 + 0.008 |
0.076 + 0.008 |
ism |
0.261 ±0.018* |
0.191 ±0.021 |
0.128 + 0.009 |
0.130 ±0.012 |
0.136 + 0.007 |
Pullulanase-D peptide |
|||||
IgG |
0.505 ± 0.034* |
0.1 96 ±0.021 |
0.186 ±0.015 |
0.200 ±0.014 |
0.215+0.009 |
IgA |
0.224 + 0.035* |
0.086 ±0.0 17 |
0.096*0.021 |
0.087 ±0.021 |
0.064 + 0.007 |
IgM |
0.508 + 0.035 |
0.298 ±0.027 |
0.426 ± 0.024 |
0.385 ±0.0 17 |
0.444 ±0.0 18 |
Control peptide |
|||||
IgG |
0.677 ±0.041 |
0.589 ± 0.040 |
0.602 + 0.032 |
0.668 ± 0.038 |
0.696 ± 0.028 |
IgA |
0.238 ± 0.026 |
0.207 + 0.027 |
0.208 ± 0.027 |
0.142 + 0.015 |
0.211 ±0.014 |
IgM |
0.469 ± 0.027 |
0.441 ±0.034 |
0.474 ±0.031 |
0.454 ± 0.034 |
0.487 ± 0.022 |
* P < 0.001 compared to controls.
111
. ft al: Klebsiella, pmteux and AS, RA
(2.)
(2b)
PULLULANASE-D PEPTIDE
HLA-B27 PEPTIDE
-7
OD
·4
OD
-4-
·2-
·1-
T
-££ :~±
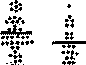
·1'
Controls Active Probably Active inactive
RA active AS AS
Controls Active Probably Active Inactive
RA active AS AS
RA
P<0-OO1
P«=0-001 P«=0-001
(2C)
CONTROL PEPTIDE
1-4
1-2
![]()
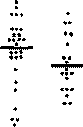
1-0
·6'
-4-
·2-
Figure 2. IgG antibodies (bars = means) in patients with RA (active and probably active), patients with AS (active and inactive) compared to control subjects, tested against HLA-B27 peptide (2A), pullulanase-D peptide (2B), and scrambled pullulanase-D control peptide (1C). The broken line represents 95% confidence limit of the distribution of the controls (significant p values compared to controls are indicated).
Active RA
Controls
Probably Active
active Ag
RA
Inactive AS
< 0.05). Furthermore, IgG (t = 10.37, p < 0.001) and IgA (t = 5.646, p < 0.001) antibodies against the pulD peptide were also found to be elevated in active patients compared to controls (Figure 2B). IgG antibodies to pulD peptide were significantly correlated with ESR (r = 0.498, p < 0.001) and CRP (r = 0.426, p < 0.01), while IgA antibodies to pulD
were significantly correlated only with ESR (r = 0.339, p < 0.05). No elevation in any class of antibody was seen against the HLA-B27 peptide or the pulD peptide in patients with RA. No antibody elevation of any class was seen against the control peptide in AS patients, RA patients, or controls (Figure 2C).
DISCUSSION
We report that Japanese patients with active AS have specific IgA antibodies to K. pneumoniae and Japanese patients with RA have specific IgG antibodies to P. mirabilis. Furthermore, IgA anti-Klebsiella antibody levels in patients with AS correlated with the degree of inflammation as measured by the acute phase reactants ESR and CRP. No such correlation was seen with anti-Proteus antibodies in RA patients; however, the group comprised only active and probably active patients and lacked patients with inactive disease, which is necessary for adequate correlation studies. This is the first report from Japan that specific antibacterial antibodies are present in patients with AS and RA. This finding confirms reports from other countries"*-'2-16-18. The significant elevation of IgA antibody tilers against K. pneumoniae in AS suggests the site of infection is the gut. The elevation in IgG antibodies but not IgA antibodies against P. mirabilis in patients with RA suggests the source of infection could be a nonmucosal site, probably the kidney. Clinically, the increased levels of occult chronic intestinal inflammation in patients with AS and urinary tract infection in patients with RA have been reported20'2'. These observations may suggest that local, subclinical bacterial infection could be related to the pathogenesis of these disorders. It has been reported that total serum IgA is elevated in AS22. However, in this study only patients with AS with active disease had significant elevations of antibodies against Klebsiella, while patients with RA had antibodies only against Proteus. These findings exclude the possibility that these results are due to a nonspecific effect of immunoglob-ulin. Furthermore, the observation of elevated anti-Proteus antibodies in patients with RA has been shown not to be due to rheumatoid factor activity23.
These relationships between AS, RA, and bacterial infections could be explained by crossreactivity and molecular similarity in amino acid sequences between human leukocyte antigens (HLA) and bacterial proteins. In 1987, Schwimmbeck, et al reported homology and crossreactivity between HLA-B*2705 and K. pneumoniae nitrogenase (QTDRED)24. Recently, a further sequence homology between HLA-B*2705 (DRED) and the terminal secretion protein of pullulanase enzyme system, pulD (DRDE), has been identified25. In our study, elevation of antibody levels against the HLA-B27 peptide and the pulD peptide in patients with AS were observed, confirming the Schwimmbeck, et al report24.
These results support the possibility that the molecular mimicry hypothesis may explain the high frequency of HLA-B27 in patients with AS. However, further studies are needed, in which each amino acid within the pulD and HLA-B27 synthetic peptides is substituted one by one to assess its importance in reactivity with test sera. In RA, molecular similarity has also been identified between protein sequences of P. mirabilis, hemolysin (ESRRAL), and HLA-
DR antigens (DRB1*0101, DRB 1*0404, DRB 1*0405 and 1402; EQRRAA)19. Additionally, a second homology has been shown between the urease enzyme (IRRET) of P. mirabilis and type XI collagen (LRREI)26.
Longitudinal studies are required to assess whether antibiotic intervention or diet therapies will reduce the specific bacterial antibody levels, thereby affecting the acute phase reactants in patients with AS and RA and slowing down the progression of arthritic disease.